Monday, December 31, 2007
Thursday, December 27, 2007
7.3 Aji-no-moto
 Aji-no-moto, the "essence of flavor", commonly known as MSG (monosodium glutamate), has been a fixture in kitchens of all sizes, all over Asia, since 1909. A few grains of the chemical turns "clear water into chicken soup" as the advertisement goes. And in fact, no ill physical effects have been noted until 1968, when reports surfaced in the US about physical discomfort suffered by some, after consuming Chinese food. Despite conflicting data from several studies later on, the label of "Chinese Restaurant Syndrome" stuck. To this day, in American culture, any discomfort, including postprandial stupor, from eating out, is automatically attributed to CRS. And restaurants began to offer MSG-free meals since that time, even though the replacement, the soup stock, is quite rich in MSG.
Aji-no-moto, the "essence of flavor", commonly known as MSG (monosodium glutamate), has been a fixture in kitchens of all sizes, all over Asia, since 1909. A few grains of the chemical turns "clear water into chicken soup" as the advertisement goes. And in fact, no ill physical effects have been noted until 1968, when reports surfaced in the US about physical discomfort suffered by some, after consuming Chinese food. Despite conflicting data from several studies later on, the label of "Chinese Restaurant Syndrome" stuck. To this day, in American culture, any discomfort, including postprandial stupor, from eating out, is automatically attributed to CRS. And restaurants began to offer MSG-free meals since that time, even though the replacement, the soup stock, is quite rich in MSG.Generally, the symptoms are mild that include transient headaches, flushing and sweating. It is unknown if some patients are ultra-sensitive to even a small dose of MSG; although it does not make sense why any reasonable immune system should attack glutamic acid, which is actually one of the 20+ essential amino acids, the building blocks of protein - unless it is something neurological as some have noticed later (see below). In any case, a normal dose of vitamin B6 seems effective in preventing or staving off CRS.
What's MSG, or more accurately, glutamic acid, got to do with the eye? Let's make a long story suitably short:
Glutamate is actually a major excitatory neurotransmitter in the brain. It is involved in the very complex behavior of NMDA (N-Methyl-D-Aspartate) glutamate receptors. It turns out that excessive glutamate can damage these receptors. In fact, excitotoxicity due to glutamic acid has been proposed to be the cause of neuron destruction in patients with strokes (and other forms of brain ischemia).
Maybe this toxicity can explain the CRS? No, not really, because there is a blood-brain barrier, aji-no-moto cannot enter the brain without invitation/breach. The source of the endogenous glutamate is the injured local cells, causing excessive neuronal excitation of their neighbors, hence the increasing tissue damage. The real culprit of CRS still remains unknown; although it is most likely not dietary MSG.
But wait, since someone has mentioned ischemia and the retina is part of the central nervous system, i.e., the brain, doesn't it make sense that glaucoma/diabetes related damages to the retina also can be blamed on glutamate excitotoxicity? Maybe so. And a lot of research projects are examining all facets of this issue. Already neuro-protection is a topic of major interest in the management of POAG. For example, drugs for treating Parkinson's disease are being used/tested in a number of advanced cases.
On the other hand, it is also very important to realize that as part of the blood-brain barrier, there is the blood-ocular barrier, which prevents the entry of exogenous glutamate (e.g., MSG in Chinese food) into the retina. And even in the lab, an extremely high concentration of glutamate is needed to produce direct cellular damages to the retina. Again, we can rule out dietary MSG in all ischemia-related retinopathies.
Often in the lay press, a few keywords are lumped together to create a seemingly scientific report. In the above case, putting MSG and neurotoxicity together in an uncritically written article can lead the reader to erroneously conclude that "eating out at a Chinese restaurant can damage your brain/retina". On the other hand, without the press coverage and the ensuing publicity, research on glutamate and its excitotoxicity would not have advanced so far. Ultimately, the patients benefit, so the system does work despite its many flaws. Readers must still exercise their own judgment, of course.
Monday, December 24, 2007
Sunday, December 23, 2007
7.2 Harder who?
 Next time, when you visit your eye doctor, casually mention that you would like to have your Harderian gland checked. The doctor will look at you quizzically, "Harder who?"
Next time, when you visit your eye doctor, casually mention that you would like to have your Harderian gland checked. The doctor will look at you quizzically, "Harder who?"With the long and rich history of human anatomy, you'd think that everything in the human body, big and small, young and old, have all been discovered and carefully documented. Not so. The Harderian gland has escaped attention, even though its existence in other mammals has long been known. In fact, it was first described in 1694 by an obstetrics professor in Basel, Johann Jacob Harder (1656-1711).
Indeed, one time, an excited nuclear medicine research group, who have just developed PET microscopy, informed us a large uptake of a certain compound (we are sworn to secrecy as to its identity) by the posterior portion of the eye, in a living rat. And they have traced it to the Gland of Harder. This uptake was later confirmed and quantified with another study. The excitement, however, was somewhat dampened when told that there was no such gland in the humans, at least not the ones we saw. In our collective knowledge, only one person recalled reading about this gland in non-primate mammals - in the comparative anatomy chapter of Wolfe's Anatomy of the Eye. This gland is hiding posterior to and underneath the eye ball, blended into the fatty tissues of the orbit. Easily missed if you are not looking for it intentionally.
What a shame - as PET (Positron Emission Tomography) microscopy is nothing to sneeze at. PET scans usually show low-resolution whole-body/brain images, to see images of a 3-mm rat eye is no small feat.
In 2006, two biologists in Pennsylvania finally published a paper: "Primate Harderian gland: Does it really exist?" in Annals of Anatomy - Anatomischer Anzeiger Volume 188, Issue 4, 3 July 2006, Pages 319-327. The finding? Indeed the Harderian gland is found in fetal and neonatal stages in humans but is largely absent from adults. And of course, its role is still unknown. (In contrast, numerous functions have been proposed for the Harderian gland in non-primates.)
Who knows why the humans do not have a functioning Gland of Harder, but then we don't have the nictating membrane, either.
Sometimes we run into an unknown. And to have found the answer is like discovering some new species in the deep jungles of Indonesia. Sometimes, however, the findings are to be filed away perhaps for another day. All part of the fun and game of research.
Friday, December 21, 2007
7.1 Cold fish eye
 A patient once remarked that her post-LASIK vision seemed to change for the worse but only during the winter time. An examination and a comparison with past records indeed confirmed her self-observation. Since no other similar reports have surfaced, we have regarded this case as anecdotal. Although, it is entirely possible that structural change of a thinner cornea in response to a lower ambient temperature did occur. It'll be interesting to study post-LASIK Alaskans' visual stability, for example.
A patient once remarked that her post-LASIK vision seemed to change for the worse but only during the winter time. An examination and a comparison with past records indeed confirmed her self-observation. Since no other similar reports have surfaced, we have regarded this case as anecdotal. Although, it is entirely possible that structural change of a thinner cornea in response to a lower ambient temperature did occur. It'll be interesting to study post-LASIK Alaskans' visual stability, for example.So how does the eyes keep warm - or cool, for that matter. Human corneal temperature is normally 34.2°C. Then there is a temperature gradient from the cornea all the way deep into the orbit, reaching the body temp. This indicates that the anterior segment, including the cornea, iris, and the crystalline lens, is kept cool through blood and aqueous circulation. Normal corneas seem to be quite comfortable with the temperature change and can stay transparent. Even solar keratitis affects mostly only the epithelium, the rest is still clear.
The crystalline lens is a different story. In a simulation, when a model human eye is exposed to an infrared radiation source of 1500°C, the lens temperature increase can reach 1-2°C, which seems enough to cause infrared cataracts.
Hmm, what if you live in a cold environment? There are the experimental "cold cataracts" seen in the lenses of newborn cows, rats, and mice; although, luckily, not in that of human babies. What about fish living in the -2°C Arctic/Antarctic ocean. Well, they have a glycoprotein antifreeze in their body systems and, most interestingly, cold-resistant lens proteins. These proteins are actually gamma-crystallins. It seems that in some cold-adapted fish, these crystallins are more tightly packed than the mammalian kinds, and are therefore far more resistant to protein-water phase separation (hence the opacities). That does make sense, or the chance of survival for a cataractous fish would not be so great.
Next time, when you look at a fish in the eye, do remember the remarkable biochemistry contained within.
What if you are an eagle flying up in the cold air and needs to hunt for a rabbit a mile away? The eagles do have an extra eyelid, as that in a rabbit, called nictating membrane. In the eagles, this membrane is transparent which swings into action during hunt. Their retinal cell density is about 5 times that of the humans. So we can safely assume the eagles have much better visual acuity than the humans. Cold cataracts? Not a chance. In the eagle eye, within the vitreous, there is a structure called pecten body. It is a vascularized feathery structure with the function of a heat radiator. That is how the eagle eye is kept warm even flying through frigid air, and, some say, pecten body also provides oxygen to the ocular structures. It turns out that all birds, including the lowly chicken, have it, too. Surprise, surprise.
It pays to strike up a conversation with a veterinarian sometimes.
Wednesday, December 19, 2007
6.6 Unexpectedly...
In fact, very often the surgical procedure itself is described as being a big success, but then things start to go wrong. In very general terms, a quick description of some (not all) post-op problems is shown below:
1. Refractive surgery
Take, for example, LASIK:
Obviously if the correction is inaccurate, e.g., under- or over-correction, residual astigmatism, or regression, then the post-op vision won't be as good as advertised.
There are also glare, haze, and halo that can result from off-centered ablation, pupil size larger than the ablated zone, or folds in the flap.
Post-op dry eye, epithelial growth, inflammation, and infections are all possibilities.
2. Cataract extraction
Cataract surgery has a 95% success rate. It is still possible to develop catastrophic infection (known as endophthalmitis), intraocular hemorrhage, cystoid macular edema, or even retinal detachment.
3. Glaucoma shunts
After previously failed glaucoma surgeries, the last resort is a shunt implant. The principal complication after implanting the shunts is hypotony (low intraocular pressure). Healthy eyes will produce enough aqueous humor to fill up the shunt reservoirs. And the eye then re-pressurizes. However, choroidal detachments or hemorrhages can occur if the eye is not healthy (e.g., from diabetes).
4. Vitrectomy
Most patients experience improved vision after vitrectomy. However, about 18% of patients develop complications with half of them having zero improvement in vision, and the other half, actually permanent vision loss from, e.g., neovascular glaucoma (the main reason why the glaucoma shunts are needed).
5. EOM re-alignment (strabismus surgery)
The most common complications are under- and over-correction, so the eyes are still not straight post-operatively. Sometimes these are transient, but other times, another surgical correction is needed. Other complications include:
Perforation of the sclera
Lost and slipped muscles
Infection
Anterior segment ischemia (from damage to the ciliary arteries)
Diplopia
Conjunctival granulomas and cysts
6. Corneal transplant
As in all transplants, the biggest problem for cornea transplant is tissue rejection. This is treated with immunosuppressants at the early stage. Other possible complications include infection, hemorrhage, retina detachment, and glaucoma. Still other problems are optical, for example, irregular astigmatism can be a result. Irregular astigmatism cannot be corrected with spectacles at all; only RGP hard contacts can cover up the irregularity.
Fortunately, most if not all of the above post-op complications can be successfully repaired or treated; although the outcome will be somewhat less than that expected before the surgery.
Tuesday, December 18, 2007
6.5 Vision rehab setup
The definition of low vision is, however, rapidly changing. It no longer denotes legal blindness or worse, rather it now includes patients with BCVA of 20/80 or less and whose vision cannot be further restored. For example, patients with cataracts plus AMD or diabetic retinopathy, or patients with post-RK ghosting and/or polypolia. Thus low vision cases are now often seen in a primary care office.
A common misconception is that since the patients cannot achieve 20/20 vision, the accuracy of refraction is no longer crucial. In fact, having lost some vision, the patients are extremely sensitive to changes, however small. Any loss is potentially devastating and gain a cause for celebration. Part of this maybe psychological; however, the change can often be attributed to the development of another sensitized areas (outside of the macula/fovea) for vision. It is the doctor's responsibility to identify these areas and maximize the patients’ vision accordingly.
The ultimate goal of low vision care is really the improvement of the patient’s quality of life. For some patents, it is also crucial to integrate vision rehab with occupational therapy, so that they can be productive again.
Low vision care is an extension of refraction including contact lens application. The main principle is magnification without compromising the available field of view. In other words, if the magnification is such that it allows the patient to read, but only one word or even only one character at a time, then it will be very difficult to scan the whole line of text, let alone reading at a reasonable speed.
The availability of low vision devices has also increased from optical magnifiers and telescopes to computerized readers, self-focusing telescopes, satellite-guided navigators, all the way to macular relocation, implanted telescopes, and ocular and cerebral electro-implants. The increasingly bionic approach can benefit patients with profound loss of vision, whether it can serve or is accepted by low vision patients remains to be seen.
Here, we will describe a typical set up of a low vision care office:
Patient flow: Wherever applicable, Braille signs must be posted. For example, in the elevators, Braille floor number signs must be posted next to the push-buttons. Also, Braille directions to the clinic should be affixed onto the walls at waist level along the way to the clinic. The clinic also must be wheel-chair accessible. The patient then enters the front desk and waiting area and proceeds to register. Pre-test room: An Ophthlamic Technician should then take history, both visual and medical. Especially important is previous history of eye treatments and/or surgeries. Previous records are important, in fact, crucial for further evaluation. Record patients’ current low vision devices and their effectiveness. And what the patients’ expectations for the visit.
Visual acuities, both aided and unaided and most important pin-hole acuity must then be taken. This is done with standard Snellen charts or equivalent. Patients should NOT be dilated at this point. Dilate only if the patient needs a retinal exam. Also, no applanation/indentation tonometry (this may distort the cornea), save this for later. Patients with POAG and silicone oil must have tonometry before conclusion of each visit.
Do auto-refraction/keratometry (note the shape of the mires) if practical. A hand-held auto-refractor may be needed for children.
Visual analysis/eye exam room: A skilled refractionist then evaluates the patients. For this room, in addition to the standard Snellen charts, a low-vision chart designed for 1m testing distance is required. For children, a childrens’ chart for 1-3m will suffice. For infants, use a separate preferential looking chart.
Accurate refraction is key to successful management of low vision cases. The following equipment is required:
Phoropter with chair and stand together with a slit-lamp
Complete set of trial lenses (including prisms) and a trial frame
Retinoscope with halogen lamp
Direct opthalmoscope with halogen lamp
Binocular indirect ophthalmoscope
Volk lenses (at least one +78D lens)
Complete set of LV trials (for example, that from Eschenbach)
Record best correctable vision and reasons for sub-optimal vision. Propose low vision aids and/or further tests.
Low vision aids: for example, telescopes, microscopes
Special optical aids: for example, prism for eccentric viewing or avoiding diplopia
Specialty contact lenses that include lenses for aphakia, aniridia, keratoconus, complications after corneal transplants or refractive surgery, contact-lens telescope
Electronic devices: for example, desk or head-mounted CCTVs
Special testing room: This room should be equipped with a special visual field testing instrument, ideally a scanning laser ophthalmoscope. In severe visual loss, SLO provides information on areas of preferential viewing and the angle of deviation from the macula, so that the amount and axis of prism correction can be determined.
A high percentage of patients suffer from glare sensitivity. A glare testing device is also needed in order to quantify the need for shields.
Education room: An ophthalmic assistant teaches the patients how to use the low vision aids. Most patients will require instructions or the devices often go unused.
Occupational therapy room: For those with severe loss of vision, an Occupational Therapist teaches assistive living with the goal of achieving independence. This room is equipment with a kitchen with utensils designed for LV patients.
A computer area for learning skills for navigating the Internet and improving office productivity.
For the immediate future, assistive technologies should be developed for the use of ATMs, PDAs, and cell phones. These are, not surprisingly, what the low-visoin patients have the most difficulty with at present time. Some never even have the opportunity to access these devices.
6.4 (Non-)Contacts
 Strictly speaking, a contact lens (CL) is not in direct contact with the corneal epithelium. Instead, it floats on top of the tear film. This is, however, only academic.
Strictly speaking, a contact lens (CL) is not in direct contact with the corneal epithelium. Instead, it floats on top of the tear film. This is, however, only academic.There are simple steps for fitting contacts:
1. Corneal evaluation:
Maintaining corneal health remains the most important goal in contact lens fitting. It is now known that the corneal endothelium can change its cell density, size and shape after contact lens wear. The endothelium is responsible for the maintenance of water content in the cornea (too much water causes corneal edema hence haze which occurs in eyes with improperly fitted CLs). Healthy cornea of course is a must for the eye to receive the CL. Eyes with corneal defects such as the dry eye will be incompatible with CL wear. The epithelial defects can be readily seen with fluorescein staining when observed under the cobalt (blue) light. Specular microscopy is needed for the evaluation of the endothelium; although this is not commonly done.
2. Parameters needed for CL fitting:
(1) The most important one is corneal curvature measured with keratometry. The curvature in mm (known as the “K”) is the basis for the selection of CLs. In other words, the base curve of the CL must be based on that of the cornea. For example, in hard lens fitting, the base curve can be on mixed K, on flat K, or slightly flatter than the flattest K, and in soft lens fitting, the base-curve is usually flatter than the flattest K. This allows CL movement that facilitates tear (essentially oxygen and nutrient) exchange. It should always be remembered that CLs are NEVER fitted steeper than K as this will cause “central pooling” (immobile CL with tears trapped underneath) leading very quickly to corneal damage. It also should be noted that the K-readings are those of the central 2-3 mm of the cornea. Trial lens fitting therefore remains the most practical approach in the selection of CL base curve.
(2) The palpebral fissure or the gap between the upper and lower eyelids when the eyes are in the normal open position can be a determining factor in the selection of lens diameter (i.e., size). This is based on the assumption that the portion of the cornea covered by the lids does not receive enough oxygen already, larger-diameter lens therefore will deprive the cornea of even more oxygen.
(3) The corneal diameter also can be a determining factor in the selection of CL size. Again, this is based on the area of corneal coverage by the CL. In general, the smaller the CL diameter the better for cornea health. Furthermore, smaller CL diameter has the same effect as increasing the base curve, i.e., smaller CLs will appear flatter than large CLs. However, it also should be noted that sometimes the patients will experience glare and unstable vision because the CL diameter is too small. And by the same token, eyes with extremely large pupils also will experience peripheral disturbances if the CL is too small.
(4) In addition to CL base curve and diameter, it is also necessary to adjust the lens power because of the difference in the vertex distance (which is the distance between the back of the spectacle lens and the cornea/eye). Notice that refraction either with the trial frame or with the phoropter, is done at a distance away from the eye of around 13 mm. The CLs are fitted directly onto the eye, so the power actually needed will be less minus (or more plus) especially when the spectacle lens power is more than 4 diopters. For example, a spectacle lens power of -5.00D requires a CL power of –4.50D. A conversion chart or software is generally available online.
Notice in the very high myopia (malignant myopia) and hyperopia (pseudophakics), the adjustments may have to be made through over-refraction as even slight change in the vertex distance can cause large differences.
3. CL Selection:
(1) Soft lenses are HEMA-based that contain 30-70% water. There are now daily wear, 3-month extended wear, daily disposable, and 1-2 weekly disposable lenses. Selection is based more on the patient’s life style and sometimes on economic concerns.
(2) Hard lenses are now silicone-based RGP lenses. The classical PMMA lens is no longer or very seldom in use. The fitting principle for RGP lenses is the same as that developed for the PMMA lens, i.e., fitting on K or more frequently, flatter than K to allow CL movements. However, because of the high oxygen transmission, RGPs can be fitted on-K for better stability. (3) Special lenses are those with toric design as well as the bifocals. They are also available in hard- and soft-forms. Because of the necessity to limit lens rotation from blinking motion, a prism ballast or similar must be built-in to anchor the lens in a fixed orientation. Nonetheless, lens rotation sometimes is still too extensive to allow stable vision. There is a momentary blur after each blink that some patients cannot seem to tolerate at all.
4. Situations that require extensive management:
(1) Dry eye – deficient tear production and poor tear quality
(2) Severe allergic conjunctivitis – chronic inflammation and discomfort (“itchy” eyes)
(3) Pregnancy and use of birth control pills – these change tear chemistry that can reduce the wear time
(4) Diabetes mellitus – general intolerance to ischemia
(5) POAG – CL wear may mask glaucoma-related corneal change
(6) Chronic and acute infectious keratitis
5. Complications from CL wear:
(1) Punctate stains – epithelial defects due to desiccation and/or oxygen deprivation often in the 3-9 o’clock position
(2) Corneal edema – from severe oxygen deficit detectable by limbal trans-illumination as central corneal clouding (ccc)
(3) GPC (giant papillary conjunctivitis) – response to allergens embedded in the CL; cobble-stone type
(4) Infectious keratitis – improper wear habit (often over-wear and poor CL hygiene) causing breakdown of corneal resistance to micro-organisms, mostly bacteria (e.g., Pseudomonas aruginosa)
6.Remedies for problems in CL wear:
(1) Proper blinking pattern and adequate blinking rate
(2) Use of wetting solutions and lubricants
(3) Shorten CL wear time
(4) Proper cleaning and disinfecting of contact lenses
(5) Use alternative types of CLs (CAUTION: hard CL re-fitting is quite difficult especially if the patient is a long-term CL wearer. It often necessitates suspension of CL wear for 3 months before the re-fit.)
Conclusions:
From the doctors' point of view, Good Vision, Ocular Comfort, plus Adequate Lens Movement are the three hallmarks of a successful contact lens fit. However, it should be noted that thorough dispensing/teaching sessions and periodic follow-ups are also required to ensure maintenance of the patients’ corneal health.
6.3 20/20 or bust
The techniques described below are collectively known as refraction. A proficient and experienced refractionist usually is the one able to provide the patients with the best Rx in the least amount of time. And the equipment is simply a retinoscope plus a trial lens set or a phoropter - or more conveniently, an auto-refractor for objective refraction.
There are automated visual analysis systems that perform both objective and subjection refraction at the same time. These instruments, however, have not become popular and are not commonly available. Most refraction is still done manually, first objective, then subjective:
1. Objective refraction
Objective refraction does not require the patient’s response. It is especially useful for the cycloplegic refraction of apprehensive babies and uncooperative children. The tools needed are (1) a trial frame with trial lenses or more conveniently, a phoropter; and (2) a streak retinoscope (or a less frequently used spot retinoscope).
The brightness of the retinoscope light source must be adequate as dim lights do not allow accurate assessment of the pupillary light reflex. The batteries (in DC models) and the light bulbs therefore must be checked and replaced regularly. In general, halogen bulbs give more intense lights hence are preferred. The best available retinoscope to date remains the Copeland streak model.
Regardless of the refractive error, the starting lens is always the +1.50D lens. The ideal working distance is 66cm (distance between the refractionist’s eye and the patient’s eye spaced by the fully extended refractionist’s arm to reach the phoropter or the trial lens), power deduction is therefore 1.50D. Shine the retinoscope light into the patient's pupil and move the scope side to side (i.e., by rotating the handle slightly). The refractionist’s right eye is used to refract the patient’s right eye and the left for left. Watch for the change in light reflex: if against motion, add minus power (or reduce plus power) and if with motion, do the reverse. The endpoint is when the reflex motion is neutral which is often when the brightness is the greatest.
In general,
(1) Do the primary meridian first (i.e., the least minus direction) using spherical lenses, then use cylindrical lenses for secondary meridian refraction. These two meridians are perpendicular to each other.
(2) Go from over-plus, then reduce the power in order to avoid accommodation especially in children – this applies to cycloplegic refraction as well.
(3) Occasionally, there are unusual reflexes, e.g., the so-called “scissors” motion, that occur especially in fully dilated pupils. In this case, look for reflexes in the central portion of the pupil and ignore the peripheral reflexes.
(4) Deduct 1.50D to reach the preliminary Rx.
The advent of auto-refractors has greatly simplified objective refraction. These instruments are in general quite accurate except in the presence of, e.g.
(1) excessive accommodation, especially in children,
(2) mydriatic pupils causing spherical aberration,
(3) unstable fixation, and
(4) opacities in ocular media or silicone oil/gas bubble in the vitreous - for these cases, retinoscopy is far more informative and accurate.
For now, auto-refractor readings, as that from retinoscopy, are used as the starting point for subjective refraction. The skill of retinoscopy itself always will be needed especially in the case of over-refraction, in pediatric cases, and when a reliable auto-refractor is unavailable.
2. Subjective refraction
It is possible to perform subjective refraction without data from retinoscopy or auto-refraction, but the process will be slow and the results not as accurate. This is especially true when refracting high astigmats (for example, >2.5D cylinder) because the spherical equivalents can mask this type of refractive error. Also, the tendency to over-minus is great, because the myopic eye can accept unnecessary minus power yet still with good acuity. Over-minus can stimulate accommodation hence are undesirable as it often leads to headaches and can potentially promote myopia progression in children. A good alternative is to use the patient’s own spectacle power (if it provides good visual acuity) as the starting point and then either over-refract or further refine. In addition, one can assume a 20/40 visual acuity will require a –0.75 to –1.00 D lens and 20/200 will probably need at least –2.00 D of correction. It should be noted that since subjective refraction is based on the patient’s response, it is necessary to ensure reproducibility. The refractionist must often remind the patients not to squint and to answer queries honestly.
Subjective refraction as a rule should not deviate too greatly from the objective finding or one of the two processes is in error.
(1) Refract the right eye first. Some refractionists prefer covering the eye not being examined, others prefer “fogging” (i.e., over-plus by 1.5 to 2D to reduce consensual accommodation). Children do have a tendency to over-accommodate even under cycloplegia. Fogging therefore is needed even under cycloplegia.
(2) For spherical power determination, the least-minus spherical power with the maximal visual acuity is the endpoint. The vision may not be ideal at this point if significant amount of astigmatism is present.
(3) Testing of astigmatism is based on (i) the fan chart followed by (ii) refinement with the Jackson cross-cylinder.
Principles: The patient’s perception of the clearest “spokes” on a fan chart can be used to calculate the cylindrical axis. The clearest lines at certain clock position (from 12 to 6 o’clock) times 3 = axis of astigmatism correction. For example, if the 2 to 8 o’clock line is the clearest, then the axis is 2x3=60 degrees and if 12-6 o’clock, then 6x3=180 degrees.
The Jackson cross-cylinder has both plus and minus cylinders in perpendicular. Flipping-over changes minus to plus cylinder and the reverse from plus to minus but without changing the axis. It is used for cylindrical axis and power refinements. For axis refinement, the principle is that when two cylinders of opposite signs are combined obliquely, the resulting axis is 45 degrees away from the midline of the two cylinder axes. The patient will report clearer view of the visual acuity chart when the minus axis of the cross cylinder is closer to the plus axis of the astigmatism cylinder. The endpoint is when neither looks clear when the cross cylinder is in normal and flipped-over positions. The amount of cylinder power is then adjusted by first superimposing the minus axis of the cross cylinder with that of the astigmatism cylinder and then compared the clarity with that when the cross cylinder is flipped over to the plus side. The endpoint is when both positions look equally clear. Alternatively, the cylindrical lens can be rotated until clear vision, refinement with the cross cylinder will then be much easier.
(4) Duo-chrome test. This is also known as the red-green test. A red-green filter is superimposed onto the acuity chart. Because of chromatic aberration of the eye, the red light is focused in the front and the green light in the back of the retina. Letters on the red side therefore will appear clearer when under-minused and the green side clearer when over-minused. The difference is usually around 0.25-0.50 diopter. The endpoint is when letters on both sides appear equally clear or when the red side is slightly clearer.
(5) Equalization of the two eyes: As the last test, the vision in both eyes must be equalized or the patient will complain of one eye blurrier than the other.
(6) Determination of Adds: Adds (i.e., additions) are plus power added onto the distance Rx in order to compensate for loss of accommodation in the presbyopes. It appears as the segment portion (known as the Seg) of the bifocals or the reading glasses if distance correction is not needed. The Add power can be decided by the Amplitude of Accommodation (with the push-up test) or the Negative Relative Accommodation (measurement of the tolerance to increasing minus power at 40cm). Or more practically, by using loose plus lenses (range: +0.75 to +3.00D) over the distance Rx to achieve a J1+ reading vision at a distance of 40cm or whatever the patient’s preferred reading distance is.
Some patients will need trifocals; the intermediate Add power is usually ½ of the near Add. There are also now the popular progressive bifocals; however, care must be taken to fit the frame properly or the optical center may change its position relative to the eye due, for example, to frame slippage down the nose. Then the patient will have problems seeing clearly. Also the upper border of the Seg is usually set at 1-2mm below the lower lid margin. Too high or too low will both cause discomfort, e.g., severe neck muscle strain.
(7) Additional considerations:
(i) PD (inter-Pupillary Distance): PD is the distance between the two eyes. The optical centers of the two spectacle lenses must match the PD or a prismatic effect will result. Unnecessary prisms are also a cause of eye fatigue and sometimes headaches. Measurement of the PD is usually done with a ruler. More elaborate PD-meters also are available. The near PD is usually 4mm less than the distant PD used in the fabrication of bifocals.
(ii) Phoria and duction measure the resting position of the eyes and the convergence/divergence capabilities, respectively. Usually phoria measurements are used in spectacle prescription in the form of the prism. This is done by first using prism lenses to dissociate fusion and then measure the amount of deviation in the horizontal (exo- and eso-phoria) and vertical (hyper- and hypo-phoria) meridians. The normal range is 2 prism diopters exo and 5 prism diopters eso. The tolerance for vertical phorias is almost none, any amount therefore should be corrected – done with base-up (BU) and base-down (BD) prisms. Base-out (BO) prisms are for the treatment of esophoria and base-in (BI) for exophoria. The prism power is to be divided equally for the two eyes. Notice phorias cannot be corrected with contact lenses which do not incorporate prisms at all.
(8) The final Rx should be written in the minus cylinder form in the following format:
OD: Sphere = - Cylinder x aaa degrees with BI (or BO) of b prism diopters
OS: Sphere = - Cylinder x ccc degrees with BI (or BO) of d prism diopters
OU: Add + e.ee diopters
PD: ff mm
Note: to convert minus- into plus-cylinder form, simply
1. Add sphere and cylinder = new spherical power
2. Change the sign of cylinder from - to +
3. Add/subtract 90 degrees from the axis
4. Example: -1.25=-0.75x50 is the same as -2.00=+0.75x140
Voilà!
Monday, December 17, 2007
6.2 Technological divide?
1. Auto-refractor: Most auto-refractors are now capable of both retinoscopy and keratometry. And within a few seconds, the values of both refractive error and corneal curvatures are obtained. The same data also can be acquired manually with a retinoscope and a keratometer, within a few minutes. So the time saved with the automated instrumentation may be important in a high volume practice. The quality of data is similar regardless of which way they are obtained.
On the other hand, far more data are generated with corneal topography than keratometry, the latter can examine only the central 3-4mm of the cornea and measure only the curvatures. Corneal topology, based on a reflection pattern of thousands of points of the entire cornea, is shown as a sagittal map. Which color-codes the curvatures according to their optical power. The data can be used for pre-LASIK planning as well as for fitting special contacts. And any corneal irregularities, e.g., keratoconus or distortion from scarring, can be easily seen.
2. Slit-lamp observation or biomicroscopy did not change much except for digital photographic attachments in newer models. Examination of the fundus with the slit-lamp and also that with either direct or indirect ophthalmoscopes can be done with a wide-field fundus camera, i.e., the Optomap retinal scanner (see picture below). Optomap imaging takes 0.25 sec/image and requires no pupil dilation. Subtle lesions, of course, can be further examined with dilated binocular indirect ophthalmoscopy.
In this case, the fast Optomap imaging for screening retinal lesions is far more convenient and comfortable for the patients. All primary care offices should consider using this instrument.
 3. Tonometry has not change much, either. It is either non-contact (air-puff), indentation, or applanation tonometry. Some instruments have hand-held models (an example is shown below) for cases with postural difficulties on the desktop models.
3. Tonometry has not change much, either. It is either non-contact (air-puff), indentation, or applanation tonometry. Some instruments have hand-held models (an example is shown below) for cases with postural difficulties on the desktop models. 4. Visual field examination can be done by using tangent screens or much more expeditiously with modern automated perimeters. The commonly used Humphrey visual field analyzers also come with a screening model. Which is based on the frequency doubling principle that can detect visual field loss due to M-cell neuron death (typically from glaucoma). And the examination can be done in a few minutes.
4. Visual field examination can be done by using tangent screens or much more expeditiously with modern automated perimeters. The commonly used Humphrey visual field analyzers also come with a screening model. Which is based on the frequency doubling principle that can detect visual field loss due to M-cell neuron death (typically from glaucoma). And the examination can be done in a few minutes.5. Retinal imaging has made great progress in the form of OCT (Optical Coherence Tomography). The image below is a good example:
 OCT is based on low coherence interferometry capable of sub-micron resolutions. It has two modes, time-domain and the more recent spectral-domain OCT. The latter is capable of ultra-high speed and ultra-high resolution, at 73 and 3 times that of the former, respectively. We will no doubt continue to witness the further progress of this technology.
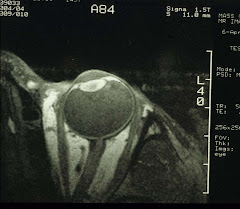
OCT is based on low coherence interferometry capable of sub-micron resolutions. It has two modes, time-domain and the more recent spectral-domain OCT. The latter is capable of ultra-high speed and ultra-high resolution, at 73 and 3 times that of the former, respectively. We will no doubt continue to witness the further progress of this technology.6. For gross anatomy of the eye, there is the old standby of office-based ultrsonography (in both A- and B-modes) and the MRI-based high-resolution surface coil imaging (see image on the rigt panel). X-Ray and CT scans for the eye are not recommended for the ionizing radiation that may cause cataracts. We favor the MRI approach as it gives far greater details than the ultrasound.
Obviously not every clinics is equipped with every piece of advanced instruments under the sun. Instruments, however, do not make diagnosis, doctors do.
Sunday, December 16, 2007
6.1 Technically speaking
Fundamentally, we need to know if the ocular functions are normal. If not, are they related to structural abnormalities that can be observed optically. And if that is not possible, then imaging with either ultrasound or radiological means. Ultimately, a decision is made with regard to treatment.
So what are the functions that must be tested?
1. Visual acuity and contrast sensitivity
Visual acuity (VA) is a measure of resolution and contrast is the ability to grade a gray scale. Contrast sensitivity is rarely tested except in specialty services.
VA is a routine test for your vision at infinity. For all practical purposes, the distance between the patient and the acuity chart is set at 20 ft or 6m, not at infinity. The rationale is that at this distance, the amount of accommodation is only 1/6=0.17D. In theory, all results of refraction must correct for this 0.17D. However, since the spectacle lenses and contacts are available only in 0.25 steps, this correction is impractical and is therefore ignored.
So what does it mean when you have 20/20 vision? It means that on the VA chart, you can see the 20/20 line at 20 ft. A person with a 20/200 vision means this person can seen the line only at 20 ft while normal-sighted people can see it 200 ft away.
There are three types of VAs: uncorrected, pinhole, and best corrected. It is usually done one eye at a time, then both eyes together. Binocular vision is better than monocular because of the psychophysical summation (i.e., two guesses reinforce each other). Pinhole acuity is, as the name implies, your vision when bypassing the optical system of the eye. It is often very close to the bested correctable vision. However, if there is no improvement with the pinholes, then it is an indication of a problem in the visual pathway.
The denotation of VA varies from one continent to another. The US is probably the lone nation holding out on the Imperial system. The table below is a conversion chart between different systems:
Snellen Notation Metric Imperial | MAR | logMAR | Decimal | |
| 6/60 | 20/200 | 10 | 1.0 | 0.10 |
| 6/48 | 20/160 | 8.0 | 0.9 | 0.13 |
| 6/38 | 20/125 | 6.3 | 0.8 | 0.16 |
| 6/30 | 20/100 | 5.0 | 0.7 | 0.20 |
| 6/24 | 20/80 | 4.0 | 0.6 | 0.25 |
| 6/19 | 20/60 | 3.2 | 0.5 | 0.32 |
| 6/15 | 20/50 | 2.5 | 0.4 | 0.40 |
| 6/12 | 20/40 | 2.0 | 0.3 | 0.50 |
| 6/9.5 | 20/30 | 1.6 | 0.2 | 0.63 |
| 6/7.5 | 20/25 | 1.25 | 0.1 | 0.80 |
| 6/6 | 20/20 | 1.00 | 0.0 | 1.00 |
| 6/4.8 | 20/16 | 0.80 | -0.1 | 1.25 |
| 6/3.8 | 20/12.5 | 0.63 | -0.2 | 1.58 |
| 6/3.0 | 20/10 | 0.50 | -0.3 | 2.00 |
The Snellen chart is the chart with letters (optotypes) and the MAR charts are designed to reflect the minimum angle of resolution, often in a logarithmic form, or LogMAR.
The design of the VA chart is based on the subtended visual angle. For example, the letters of the 20/20 line of a projected Snellen chart is usually E V O T Z 2. Look at the E:
 Each "stroke" of the E subtends an angle of 1 min, or an arc on a circle with a radius of 20 ft (6m). This one-min arc seems the minimum for the retinal photoreceptors to resolve (within the confines of the optical system of the eye and the density of the cells). All other VA charts are based on the same principle.
Each "stroke" of the E subtends an angle of 1 min, or an arc on a circle with a radius of 20 ft (6m). This one-min arc seems the minimum for the retinal photoreceptors to resolve (within the confines of the optical system of the eye and the density of the cells). All other VA charts are based on the same principle.Is it possible to see better than 20/20? Yes, in fact 20/15 is not unusual. And if the patient's eyes are designed for 20/15 vision, the endpoint of refractions cannot stop at 20/20 or the patient will still see blurred objects. The optical system of the eye is imperfect, it has a fairly large chromatic aberration. To see even better, we'll need the wavefront technology.
If we regard the light as a bundle of rays, then a line drawn perpendicular to these rays is called a wavefront. Which is flat when reaching an eye with no aberrations. Otherwise, it appears irregular. These (monochromatic) aberrations are the higher-order ones, such as coma, terfoil, spherical aberration, etc. Wavefront-guided LASIK is now the standard and indeed the vision can be corrected to better than 20/15. On the other hand, the optical application of wavefront technology, i.e., spectacles and contacts, is highly individual. And the gain probably does not justify the costs.
2. Cover and binocular tests
This is a simple test for the position of the eyes when they are dissociated - by covering one eye while the other looks (fixates) at an object. Then the cover is switched to the other eye, and the uncovered eye now re-fixates. If it moves nasally, that suggests the eye turned out when covered. This is a case of eso- phoria/-tropia. And so on and so forth.
To quickly find out if the two eyes work together, a simple Worth-4-dot test should do. The patient wears a John Lennon style specs with one green lens and the other red. The Worth-4-dots are 4 holes on the round glass plate of a flashlight (see picture below) with approximately 1cm in diameter for each hole. The holes are organized in a diamond shape. Conventionally, the top hole is red, both the left and right are green, and the bottom is white. If only one eye is functional and the other is suppressed, then the patient sees either 2 or 3 lights. If both eyes fixate together, then 4 lights. And if double vision, 5 lights.
To assess if stereopsis is present, a series of polarized images are shown to patients wearing polarized specs. The degree of stereo vision or even its absence can be readily tested.
And to see if all EOMs are working in coordination, by asking the patients to follow the movement of a penlight going horizontally, vertically, and obliquely, the EOM in trouble can be quickly identified.
3. Pupillary responses
This topic by itself can be the basis for a paperback. Let's bypass the neural pathways for now. We'll simply tabulate the abnormal pupillary responses ("+"=yes and "-"=no):
Pupil Anomalies | Anisocoria | Light reaction | Near reaction | 0.125 % pilocarpine | 4 % or 10% Cocaine | 1% hydroxy- amphetamine | 1 % pilocarpine |
Tonic pupil | (+) | (-) or minimal | (+) | (+) | No indication | No indication | No indication |
Acute third-nerve palsy | (+) | (-) | (-) | No indication | No indication | No indication | (+) |
Pharmacologic dilation | (+) | (-) | (-) | No indication | No indication | No indication | (-) |
Horner’s Syndrome | (+) | (+) | (+) | No indication | (-) | (+) 1st or 2nd order neurons | No indication |
Argyll Robertson pupils | (+) | (-) | (+) | No indication | No indication | No indication | No indication |
The pupil testing technique is knows as the swinging penlight test. Normally, the pupils of the two eyes are both round and equal in diameter. And when one eye is exposed to the bright penlight, its pupil and that of the other eye will both constrict. When the light is swung over to the other eye, both pupils will remain constricted. The pupils also constrict when looking from far to near. Anything else is not normal indicating a possible neurological deficit.
For example, if, when the penlight is swung over to the other eye, and the pupil, instead of remaining constricted, now dilates, then we have a Marcus-Gunn pupil. This suggests a lesion in the optic nerve.
And in the table above, anisocoria means unequal pupil size. Tonic pupil is usually a result of trauma. One particular form with unknown etiology, called Adie's tonic pupil, is fairly harmless often seen in young ladies. Argyll Robertson pupils are associated with neurosyphilis. And Horner's syndrome is often associated with cancer at the tip of the lungs that impinges upon the sympathetic nerve on the carotid artery.
The tests listed above are all low-tech ways that can be done easily. And they are in fact performed routinely in all clinics. The results can be quite informative, often alerting the doctors to the presence of potentially sight- or life-threatening diseases.
Saturday, December 15, 2007
5.4 People who need people
Epidemiology is a fascinating field of study. In fact, large-scale population studies are now relatively common. The conclusions are, however, confusing to the general public at times.
In 2001, the National Eye Institute sponsored AREDS (Age-Related Eye Disease Study) reported that nutritional supplements significantly reduced the progression of early AMD. These supplements included
- 500 milligrams (mg) of vitamin C
- 400 international units (IU) of vitamin E
- 15 mg of beta-carotene
- and 80 mg of zinc oxide
Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. "Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality." Ann Intern Med. 2004 Nov 10.
Some AMD patients who were on vitamin E supplements went into the panic mode, when in fact the "high-dose" in the 2004 report refers to >>400IU daily intake. The headline writers were somewhat irresponsible.
Ideally, all comparative epidemiology studies employ the same methodology so that the comparisons can be done. One good example is the cataract prevalence studies, most of which use their own home-versions of cataract classification. Your idea of a nuclear cataract-caused blindness may just be my 20/200 vision with a NS3+. So how are we going to compare the data? Eventually, the WHO organized a Cataract Grading Group who published "A Simplified Cataract Grading System" in the September, 2002 issue of Ophthalmic Epidemiology. However, to this day, the WHO system has not been widely adopted, let alone applied. Inertia is probably at the root.
It is really just common sense. For international studies, a common methodology must be agreed upon and strictly adhere to. The results will be far more useful for worldwide public health planning.
5.3 Here comes the sun
Sun/UV damage to the eye comes in many flavors. The one we still occasionally see is solar retinitis, i.e., staring at the sun with unprotected eyes for a prolonged period of time. The reason for this behavior is unknown, it certainly is a good way of burning up your fovea quickly, resulting a small central scotoma. Sometimes there is recovery from re-grouping of the cones. Most often, not.
Then there is "sunburn" of the cornea, i.e., solar/UV keratitis. The source of the UV is not necessarily the sun, it can be a sunlamp or welding arcs. Needless to say, it is quite uncomfortable.
The most prevalent UV-related damage is actually pterygium.
Pterygium is a benign fibroblast growth from the conjunctiva into the cornea (the more localized form is called pinguecula). The image below shows a mild case. Occasionally it becomes inflamed. In more serious cases, the growth can extend into the pupillary area obstructing vision. Then a surgical excision and corneal transplant maybe needed.
 In a recent study by the ophthalmology researchers at Kanazawa Medical University in Japan, pterygium appears to occur at a far higher incidence among the residents of the Island of Hainan in Southern China than the residents in Northeast China. Higher UV and ambient temperature appear the two most prominent factors.
In a recent study by the ophthalmology researchers at Kanazawa Medical University in Japan, pterygium appears to occur at a far higher incidence among the residents of the Island of Hainan in Southern China than the residents in Northeast China. Higher UV and ambient temperature appear the two most prominent factors.All of a sudden, it is a public health issue because the more extensive form of pterygium affects people, especially farmers, in all tropical areas. There are many unresolved issues: how to treat and rehab existing cases, how to prevent the occurrence of pterygium and in what way, even how to persuade the somewhat skeptical farmers to wear UV-blocking gears including eyewear.
Friday, December 14, 2007
5.2 Die Moldau
 Now, we'll go into the flow of water in the eye. From our point of view, it flows like the Vltava (the fans of Bedřich Smetana of course may disagree).
Now, we'll go into the flow of water in the eye. From our point of view, it flows like the Vltava (the fans of Bedřich Smetana of course may disagree).The traditional concept of water flow in the eye actually refers to the flow of the aqueous humor. Aqueous humor is produced by the ciliary body. It is a plasma filtrate that does not contain any blood cells (or we'd see them all day long). It enters the posterior aqueous chamber first, then passes through the pupil, i.e., between the anterior surface of the crystalline lens and the iris border, into the anterior (aqueous) chamber where it provides nutrients for the lens and the corneal endothelium. Within the aqueous chamber, there is also a temperature-mediated convection with warmer current rising to the top. The aqueous humor then exits through the angle into the trabecular meshwork and Schlemm's canal, then drains into the aqueous vein, and eventually re-enters the circulatory system. As we have explained in previous posts that any impedance along the pathway creates an increase in the intraocular pressure that may lead to damages to the retina.
To measure the flow rate of the aqueous humor, a suitable marker must be used. Since fluorescein can be delivered through the cornea into the anterior chamber, by using fluorophotometry, it is possible to determine the flow rate of this molecule (MW=332.32) and it is 2 microliter/min. This is, however, not a true measurement of the free water (MW=18.00) itself but only that of water in a confined space. It is similar to determining how fast the tap water is going through a garden hose. Free water, on the other hand, does not behave that way.
An imaging study has been done and its summary quoted below:
"Movement of water in the anterior chamber of the rabbit eye in response to topical agents for glaucoma therapy was investigated using high-resolution T2-weighted surface-coil magnetic resonance imaging (MRI) aided by a T2 contrast agent, oxygen-17 water. This water-tracer was applied topically which entered the anterior chamber within 10 min. The kinetics of its dissipation from the anterior chamber was recorded with serial T2-weighted MRI. The data represented the total water flow rate in the anterior chamber.
"We tested the effects on water flow of an alpha-adrenergic (phenylephrine), a miotic (pilocarpine), and a beta-blocker (timolol). The results showed that phenylephrine reduced more than 2/3 the normal water flow rate, pilocarpine accelerated the initial flow rate ca. 2-fold although oxygen-17 water did not fully dissipate, while timolol had little or no effect. These results suggest that water flow in the anterior chamber is in large a function of the status of the iris surface area and/or iris circulation. "
It should be noted, the flow rate using the water tracer, oxygen-17 water (MW=17.00) is 10 times that with fluorescein. In the eye, water is expected to interact with the transport systems of all tissues, not just the flow indexed by fluorescein. Indeed, we need to have a more comprehensive view of water flow in the eye.
Think "Die Moldau".
5.1 Franz Schubert Symphony No 8
Eye research will always remain unfinished, albeit in a much different sense. Schubert is long gone (1797-1828); luckily, there will always be dedicated people who try very hard to understand the eye and figure out the cure for eye diseases. So whatever is unfinished is actually an opportunity and a challenge for the newcomers.
In the US, the ongoing areas of research are defined and sponsored by the National Eye Institute/NIH, including retina, cornea, lens and cataracts, glaucoma, strabismus (plus myopia), and low vision. Each of the areas is also supported by non-profit private organizations and the instrumentation/pharmaceutical industries.
A very good example of the translation of NEI-supported research into treatment success is the anti-VGEF therapy. Because of the demonstrated efficacy in the treatment of neovascular AMD, clinical trials have now extended into other neovascular eye diseases, e.g., RAVE (rubeosis anti-VGEF trial for ischemic central retinal vein occlusion) and VISION (VGEF inhibition study in ocular neovascularization). Others are being examined as well that include diabetic macular edema, cystoid macular edema, and proliferative diabetic retinopathy.
On the other hand, we have noticed a gap between neurophysiology and impaired vision. A very versatile yet under-utilized imaging tool has been available since 1993, i.e., fMRI.
Functional magnetic resonance imaging (fMRI) permits visualization and quantitation of blood flow, volume, and oxygenation in the brain during sensory and motor stimulation. There are two variations of this technique, one detects the susceptibility effect from intravenously administered contrast agents, e.g., GdDTPA, and the other oxygen-induced susceptibility and changes in tissue diffusion but without the use of contrast agents. The non-contrast method is preferred because of its total non-invasiveness. Its applicability to brain function research, especially the photically stimulated primary visual cortex has been verified with a number of studies. Indeed, fMRI has been designed to test various visual stimuli, the studies also pave the groundwork for testing higher functions such as color vision, eye tracking and movement, retinal rivalry, suppression, and stereopsis.
An example is correlating cerebral activation with eye blinking.
 Notice the upper leftmost image is activation of the orbitofrontal lobe during normal blinking of once per 4 sec. The rest of the cerebral cortex remains quiet until when the blinking is inhibited, then even the visual cortex is activated.
Notice the upper leftmost image is activation of the orbitofrontal lobe during normal blinking of once per 4 sec. The rest of the cerebral cortex remains quiet until when the blinking is inhibited, then even the visual cortex is activated.Much more on ocular malfunctions and the brain's plasticity can be learned by using fMRI. For example, correlating fMRI results and retinal lesions can be done with point-to-point projection between fMRI and the patient's visual field defects from, e.g., open-angle glaucoma, hemifield defects, optic neuritis/atrophy, diabetic retinopathy, and AMD. Indeed, it is not even known, for example, how the primary visual cortex responds to central scotoma, let alone the understanding of brain plasticity in association with preferred retinal loci.
What about lazy eye, treatment (L-DOPA?), plasticity...
Indeed, this field is still wide open.
4.11 Broken windshields
Remember that the projection of images onto the retina requires that the cornea, the crystalline lens, and the vitreous all remain optically clear. Any opacities can cast shadows on the retina. The most obvious and commonly seen are cases of cataracts and floaters. Less obvious is corneal anomalies.
Here is one case with a chief complaint of glare. The cornea has previously undergone RK, i.e., radial keratotomy, with 8 incisions on the cornea:
 Here you can see 4 bright radiating lines. They are part of the 8 RK incisions. The cornea is thinner under each cut thereby allowing more light to pass through. This is the reason why this patient sees star burst when looking at any point source of light, e.g., headlights from the approaching automobiles. The edges of the upper left incision is also irregular and it passes over the macula - not an agreeable situation for driving at night.
Here you can see 4 bright radiating lines. They are part of the 8 RK incisions. The cornea is thinner under each cut thereby allowing more light to pass through. This is the reason why this patient sees star burst when looking at any point source of light, e.g., headlights from the approaching automobiles. The edges of the upper left incision is also irregular and it passes over the macula - not an agreeable situation for driving at night.Yet another example is the multiple dark shadows seen in the image below:
 And the source of these dark spots is shown in the following image:
And the source of these dark spots is shown in the following image: The white areas are a form of corneal dystrophy. The opacities may decrease or increase in intensity causing vision change.
The white areas are a form of corneal dystrophy. The opacities may decrease or increase in intensity causing vision change.Other types of not-so-subtle broken windshields include:
 This is a case of recurrent corneal erosion with pain and decreased vision. And in another, a very common case of superficial punctate keratitis (SPK):
This is a case of recurrent corneal erosion with pain and decreased vision. And in another, a very common case of superficial punctate keratitis (SPK): The tiny green spots are areas with epithelial defects, often appearing in very dry eyes.
The tiny green spots are areas with epithelial defects, often appearing in very dry eyes.And of course there are infiltrates from inflammation due to contact lens over-wear:
 In this case, an infiltrate slightly to the left of the pupillary area is detected.
In this case, an infiltrate slightly to the left of the pupillary area is detected.Most superficial corneal defects from infection and inflammation can be treated medically and the recovery is almost all total.
Irregular corneas that severely limit vision from, e.g., keratoconus or corneal scarring, may need to be replaced with donor corneas.
Wednesday, December 12, 2007
4.10 Itchy eyes
 Allergic conjunctivitis is quite common. It can be seasonal or perennial. It is a Type I hypersensitivity, an inflammation of the conjunctiva resulting from an immune response to allergens.
Allergic conjunctivitis is quite common. It can be seasonal or perennial. It is a Type I hypersensitivity, an inflammation of the conjunctiva resulting from an immune response to allergens.The main symptom of allergic conjunctivitis is severe itch. The eyes also look red and irritated. Very simply, the allergens (from cat dander, house mites, pollens, or chemicals, etc) sensitize the T- and B-cells to produce IgE antibodies. IgE then binds and activates the mast cells. The allergens then further cause the rupture of activated mast cells, thereby releasing histamine and other chemicals.
The itchiness is a response of nerve endings to histamine which also causes vasodilation and blood vessel leakage - hence the redness of the eyes and the formation of conjunctival follicles. So the general principle of treating allergic conjunctivitis is to avoid exposure. Failing that, to use a topical fast-acting antihistamine or a slower-acting mast cell stablizer, or even better, a combination antihistamine-mast cell stablizer. And in severe cases, to treat first with topical anti-inflammatories followed by the anti-allergy drops.
The most commonly prescribed combination eyedrops include Patanol, Zaditor, and Optivar. The newer mast cell stablizers include Alamast and Alocril. Sometimes low concentration soft steroids such as Alrex can also be used on a long-term basis. We, however, favor the combination drugs for their efficacy and safety.
4.9 Missing visual fields
It is expected that some parts of the visual fields are lost from retinal diseases such as POAG , RD and diabetic retinopathy. However, many other losses are behind the eyeball, from impairments to the visual pathway. An it can be anywhere from the optic nerve, to the optic track, the lateral geniculate nucleus, the optic radiation, and the visual cortex. Any problem along the way exhibits a unique pattern of the field loss.
In all textbooks, the lesions (indicated by a - g in the diagram below) and their corresponding field losses are depicted in a schematic such as the following:
And a more anatomically correct representation, looking at the base of the brain, is shown below:
By examining the visual fields, the location of the lesions becomes apparent. The real question is then what the nature of the lesion is. Most times it is either a tumor or some cerebrovascular diseases. An urgent referral to a capable neurologist is therefore in order.